Manganese oxide minerals are important in low-temperature surface environments and in industrial and technological applications. These minerals usually occur as very fine-grained aggregates, making it difficult in many cases to determine their structures. Studies using powder X-ray diffraction, Reitveld refinements, transmission electron microscopy, and powder infrared spectroscopy have determined the tunnel and layer framework structures of most of the Mn oxides. However, many Mn oxides also contain cations, water molecules, and hydroxyl groups within the layers and channels. The hydrous species play an important role in the structures and oxidation properties of Mn oxides, including those with the birnessite layer structure.
Powder X-ray diffraction data reveal two general categories of birnessites. The unheated Mg2+, Ca2+, and Ni2+ layer structures have 10 Å interlayer spacing, while the other cation-exchanged and natural samples have an interlayer spacing of 7.3 Å. Chalcophanite has one type of ordered interlayer water site, consistent with the well-ordered structure of defects in the Mn layers and Zn2+ in the interlayer region. The Li, Na, K, Cs, and Pb synthetic birnessites each contain two to three structurally different water sites, as evidenced by multiple H2O bending and stretching modes in the infrared spectra. The complexity of the water bands in these spectra is due to disordering of cations on the interlayer sites. H birnessite contains both water and hydronium (H3O+). The small difference in the width of the water stretching modes between room temperature and -180 °C indicates that the water molecules are structurally, rather than dynamically, disordered. There is a linear relationship between the temperature at which most of the water is lost from a given cation-exchanged birnessite and the heat of hydration of the interlayer cation. This implies that the interlayer water is strongly bound to the interlayer cations, and plays an important role in the interlayer cation exchange process.
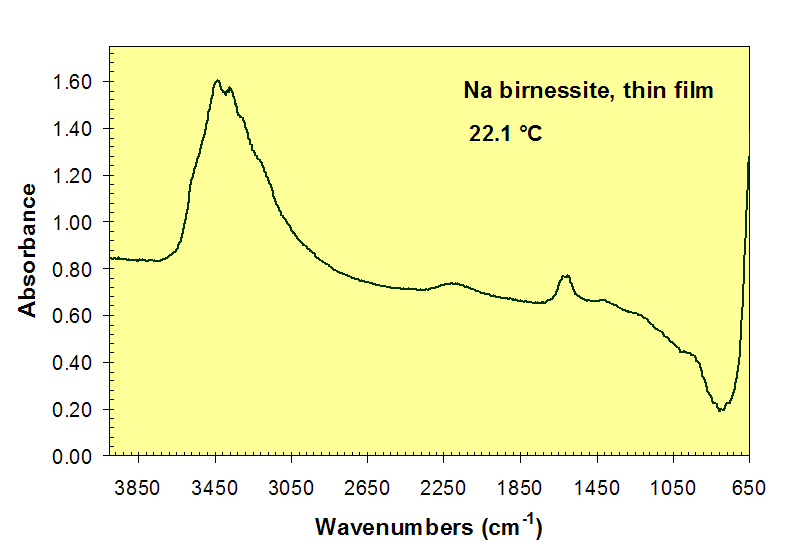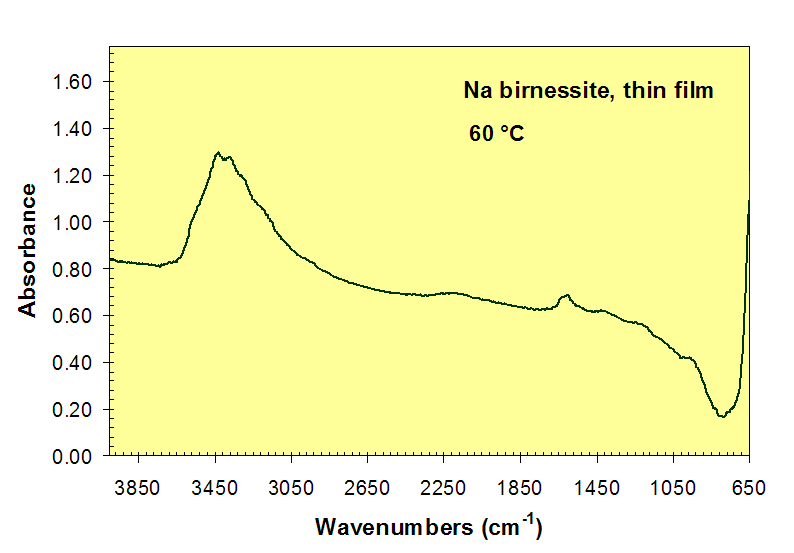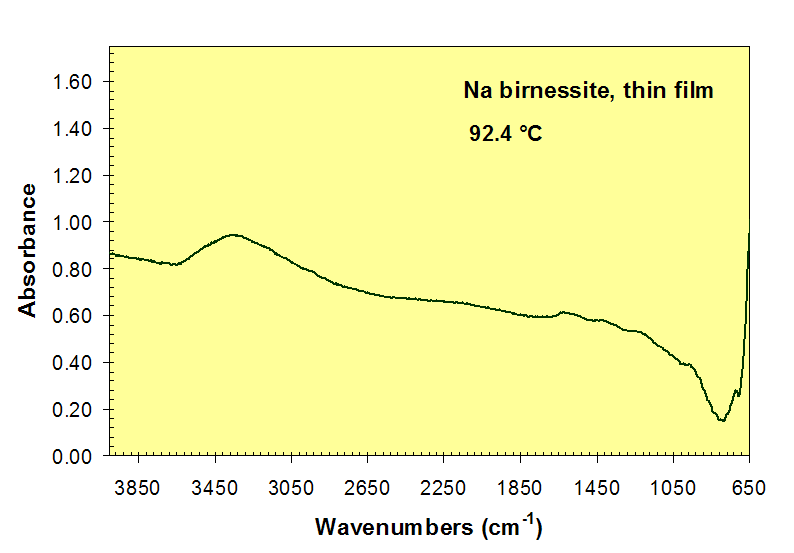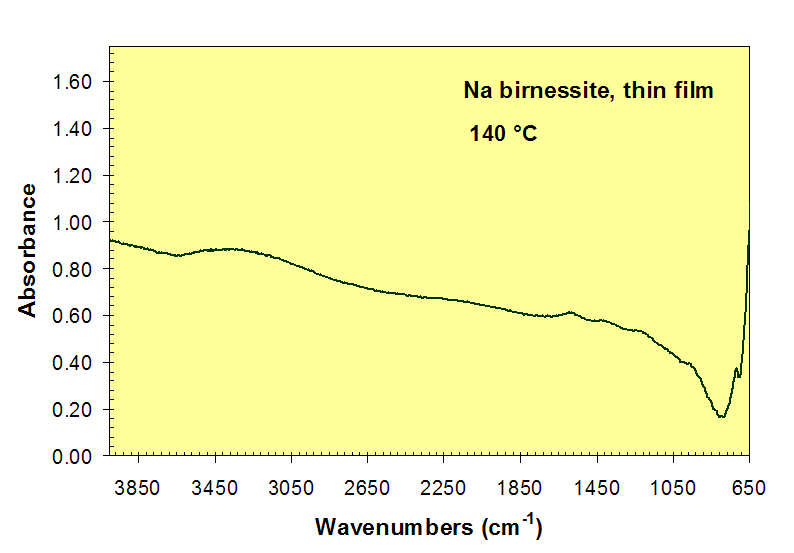
Loss of H2O absorption bands near 3450 and 1600 cm-1 during heating of Na-birnessite.