ESS 109C Isotope Geochemistry Notes
May 21, 2007
Carbon isotope geochemistry
- Class
notes & homeworks are available online Ð
http://www2.ess.ucla.edu/~schauble/Isotope_geochemistry/
- Carbon isotopes
- 12C: 98.89%, 13C: 1.11%
- Standardized indirectly to PDB (Peedee Belemnite) carbonate
- Usually measured as CO2, produced from McCrea reaction of carbonate (like 18O/16O) or oxidation of organic matter.
- McCrea reaction quantitatively releases carbon, so d13C can sometimes be measured with greater precision than d18O in carbonates.
- Fractionation during photosynthesis
- Basic reaction: CO2 + H2O ˆ (CH2O)n + O2
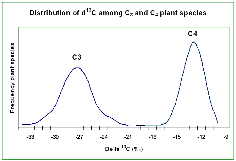
- Organic carbon is almost always 13C-depleted relative to CO2.
i. Typical atmosphere CO2, d13CVPDB Å Ð8ä
ii. Typical organic matter, d13CVPDB Å Ð25ä, but variable.
- Fractionation mechanism is a combination of kinetic and quasi-equilibrium effects:
i. Diffusion of CO2 to and through cell membranes (~pinhole diffusion). Some dependence on water stress / drought tolerance for terrestrial plants (stomatal constriction, root-leaf transport of water).
ii. Preferential reactivity of 12CO2 on fixing enzymes.
iii. Subsequent synthesis of complex organic compounds
- Net fractionation depends on photosynthetic pathway.
i. C3 plants (Calvin cycle) Ð most depleted. Most trees, bushes, some grasses). Largely passive CO2 assimilation into cell
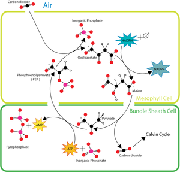
ii. C4
plants Ð least depleted. Tropical/warm-season grasses. CO2-pump based on
pyruvate ˆ
malate conversion (uses ATP/NADPH energy). Adapted to CO2
scarcity/water stress (cartoon from Wikipedia).