Characteristic Vibrations of CCl2FCClF2
(C1
or Cs symmetry)
CCl2FCClF2 (1,1,2-trichloro-1,2,2-trifluoroethane,
also known as CFC-113 or Freon 113) is similar in structure to the hydrocarbon ethane (C2H6),
with the hydrogen atoms replaced by a mixture of chlorine and fluorine
atoms. There are no known natural sources of this molecule, and
the current atmospheric abundance of ~0.084 ppb results from use by humans. CCl2FCClF2 appears to be used mainly as a solvent for cleaning and degreasing, but it may have other applications as well. CCl2FCClF2,
like the rest of the chlorofluorocarbon family (the CFC's, for short),
is relatively chemically
inert (unreactive), so it can accumulate in the atmosphere. The mean
atmospheric lifetime is approximately 85 years (Fraser et al., 1996, J. Geophys. Res. 101:12585-12599). In the stratosphere chlorofluorocarbons are fragmented by ultraviolet light, generating chlorine radicals
(Cl•) that promote the decomposition of ozone. CFC production was
phased out starting in the late 1980's. Accumulation of CCl2FCClF2 in the atmosphere is a minor contributor to the Earth's enhanced
greenhouse effect, adding a radiative forcing ≈ 0.03 Watts/m2 (IPCC).
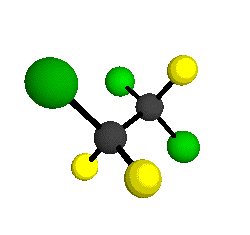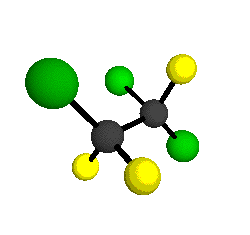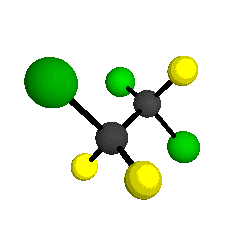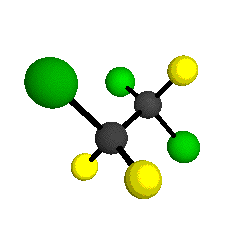
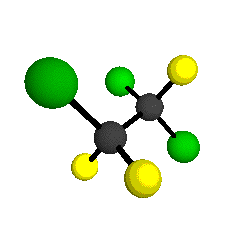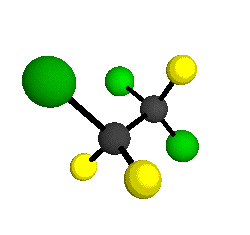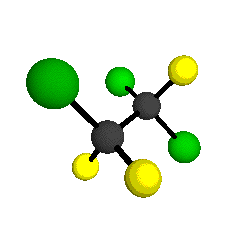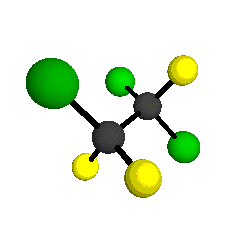
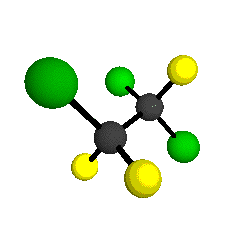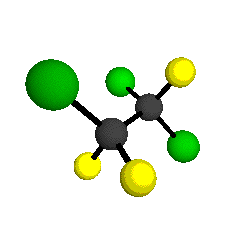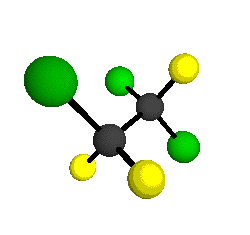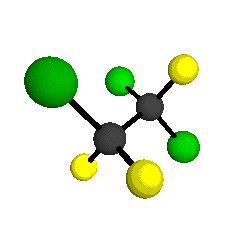
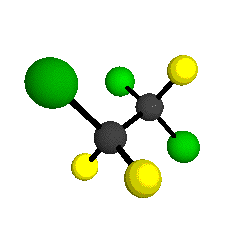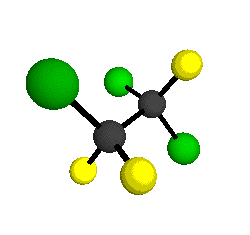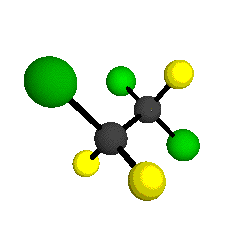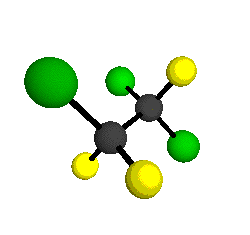
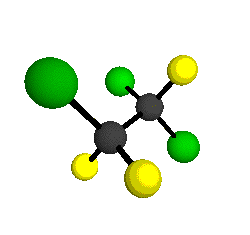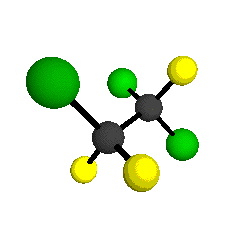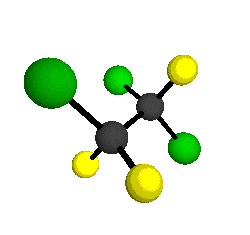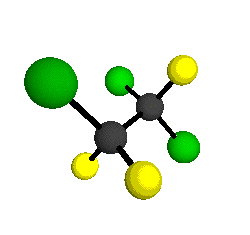
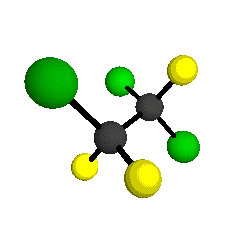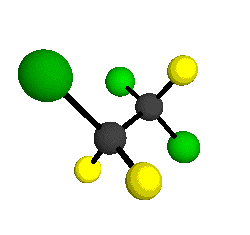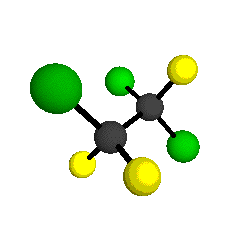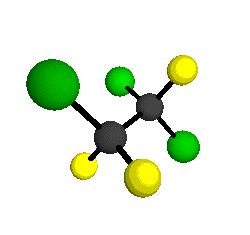
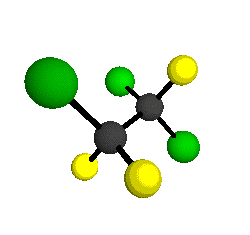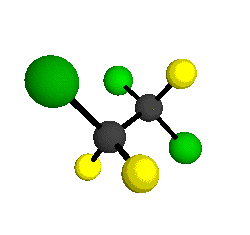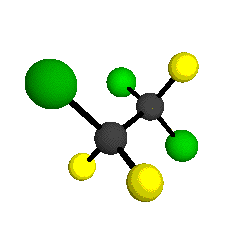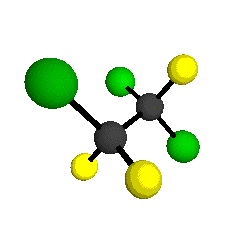
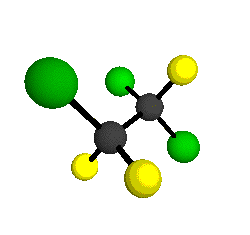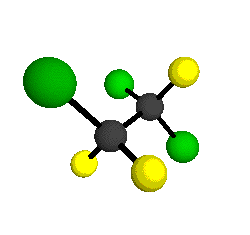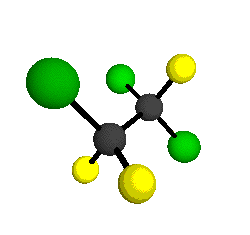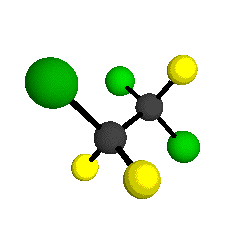
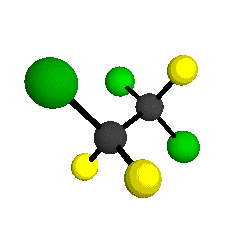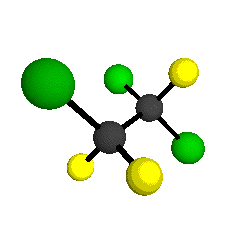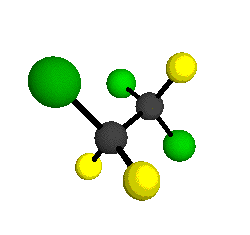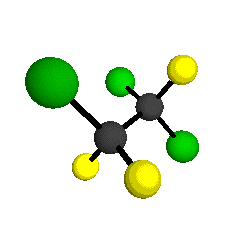
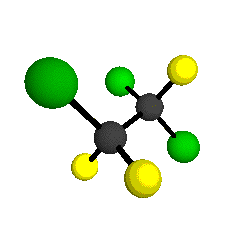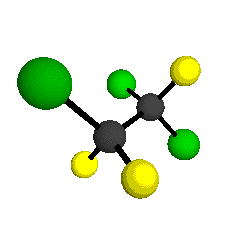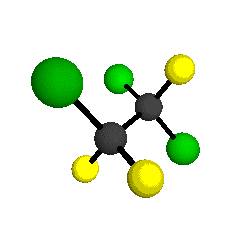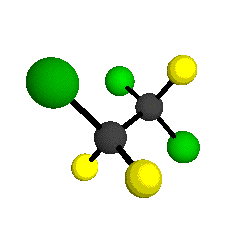
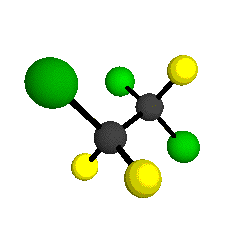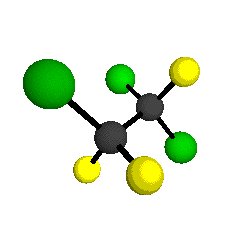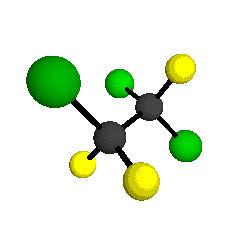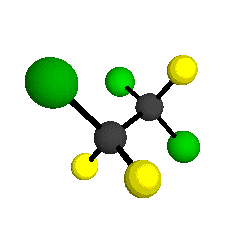
CCl2FCClF2
is more structurally complex than the 1-carbon CFC's, and can occur in a couple of different conformations, called gauche and trans. The trans
structure has a mirror-plane symmetry, with the two carbon atoms, the
single fluorine bound to the first carbon, and the single chlorine
bound to the second carbon all lying in the same plane. The gauche structure is twisted along the C-C bond axis so that it does not have any symmetry. The models shown here are of the gauche structure, which is thought to be more stable and common (Paige and Schwartz, 1992, J. Phys. Chem. 96:1702-1705). Chlorine has two common stable isotopes (35Cl - 75.77%, 37Cl - 24.23%) that are mixed more or less randomly in CCl2FCClF2
molecules, yielding a handful of isotopic forms. All of the structural
forms and isotopologues have eighteen distinct
vibrational modes, all of which interact with infrared light to some
extent. The highest-frequency vibrations are most relevant for the
greenhouse effect, because of the relatively low absorbance of other
atmospheric molecules in their frequency range. The normal
modes
depicted below were modeled using hybrid density functional theory
(B3LYP) and the cc-pVTZ basis set. Vibrational frequencies measured
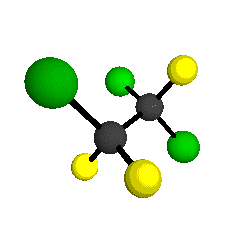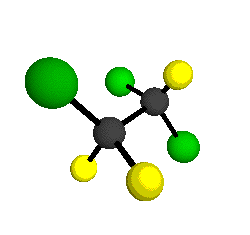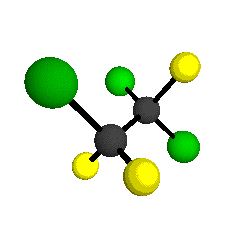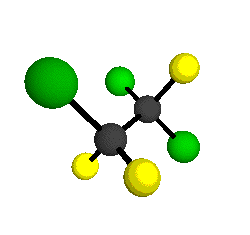
by Braathen et al. (1987, J. Mol. Structure 157:73-91) are also shown -- corresponding to a mixture of the different isotopic forms of the gauche molecular structure. Listed frequencies have not been corrected for anharmonicity. All of vibrations shown belong to the A symmetry species.
o1
= 1210 cm-1
|
o2
= 1178 cm-1
|
o3
= 1118 cm-1
|
o4 = 1047 cm-1
|
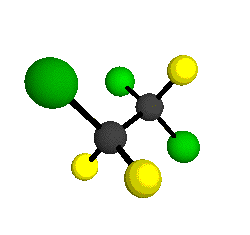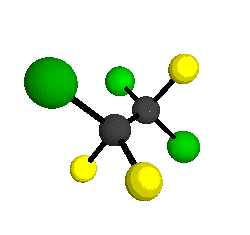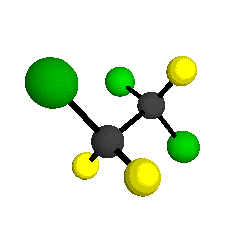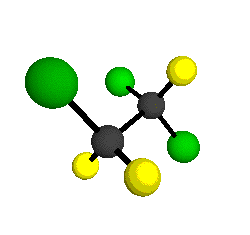 |
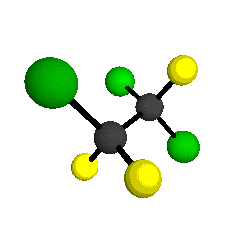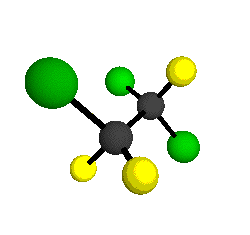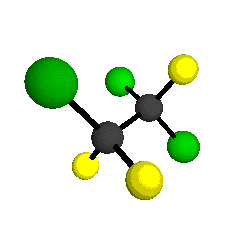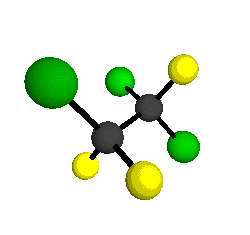 |
|
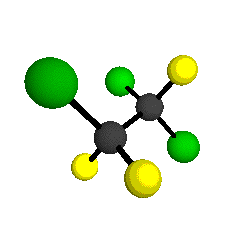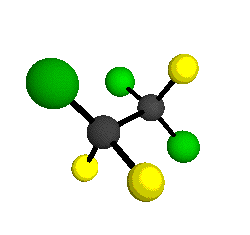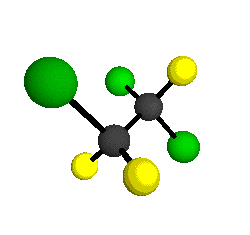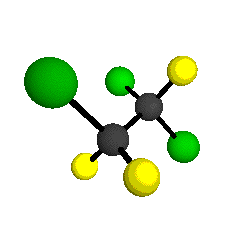 |
o5 = 903 cm-1
|
o6
= 813 cm-1
|
o7
= 654 cm-1
|
o8
= 531 cm-1
|
 |
 |
 |
 |
o9
= 460 cm-1
|
o10
= 443 cm-1
|
o11
= 392 cm-1
|
o12
= 350 cm-1
|
 |
 |
 |
 |
o13
= 315 cm-1
|
o14
= 288 cm-1
|
o15
= 240 cm-1
|
o16
= 201 cm-1
|
|
 |
|
|
|
o17
= 168 cm-1
|
o18
= 62 cm-1
|
|
|
 |
|
|
Go
to Molecular Vibrations

















