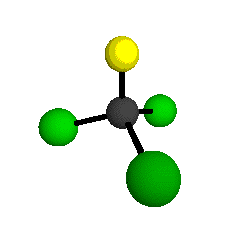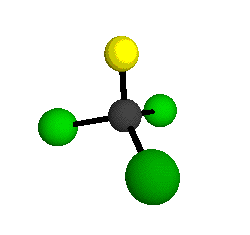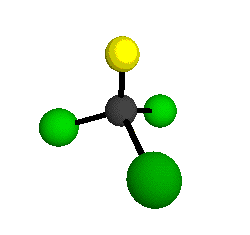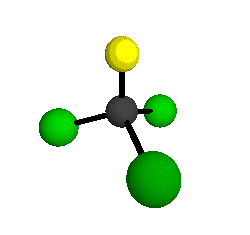
o1
= 1081.3 cm-1 (sym.)
|
o2
= 538.2 cm-1 (sym.)
|
o3
= 351.4 cm-1 (sym.)
|
 |
 |
 |
o4 = 849.5 cm-1 (sym.)
|
o5 = 399.2 cm-1 (sym.)
|
o6
= 244.1 cm-1 (sym.)
|
 |
 |
 |