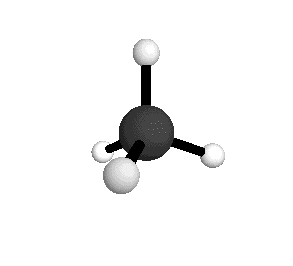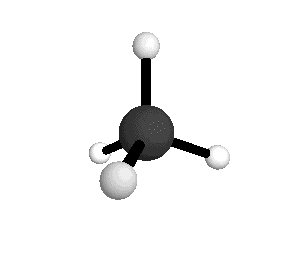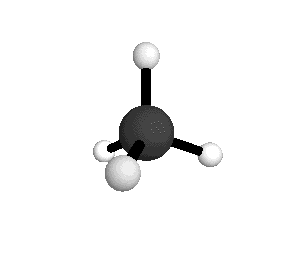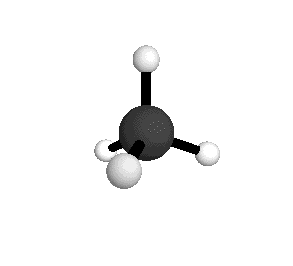
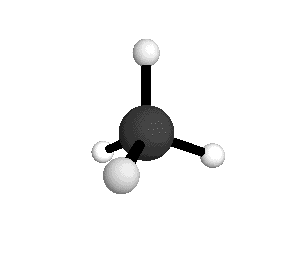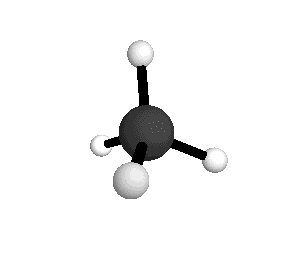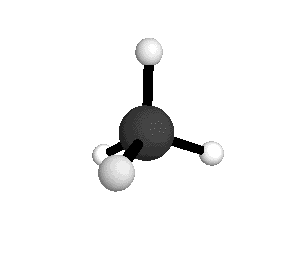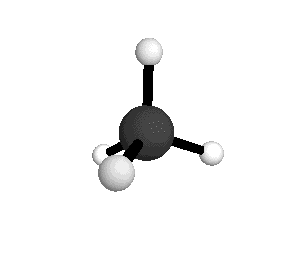
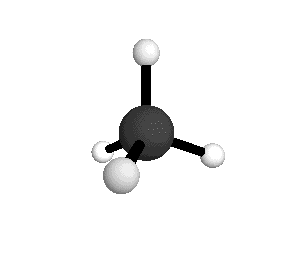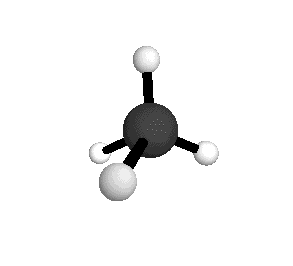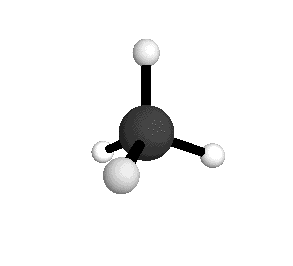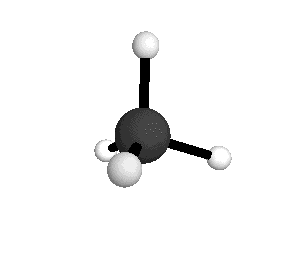
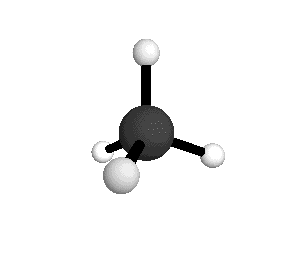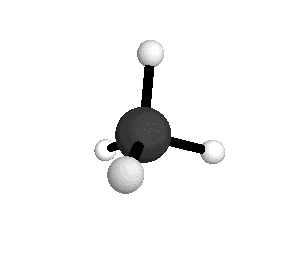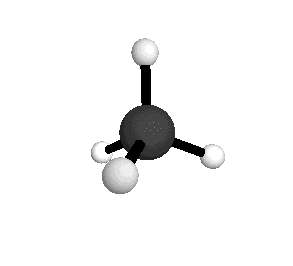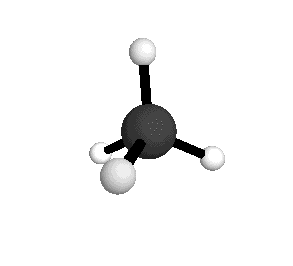
o1
= 3025.5 cm-1
|
o2
= 1582.7 cm-1
|
 |
 |
o3
= 3156.8 cm-1
|
o4
= 1367.4 cm-1
|
 |
 |