o1 (A) - Symmetric Stretch
540 cm-1 (517 cm-1)
o3 (F) - Stretch
560 cm-1 w/ 50Cr (556 cm-1)
555 cm-1 w/ 53Cr (550 cm-1)
o4? (F) - Bend
329 cm-1 (321 cm-1)
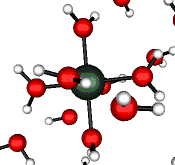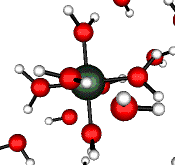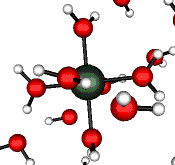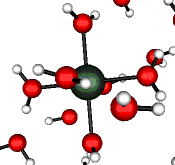
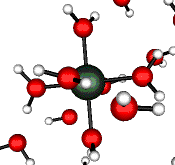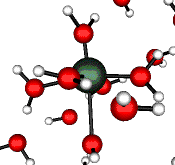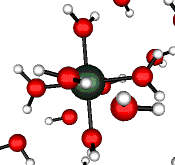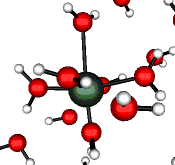
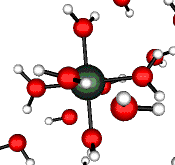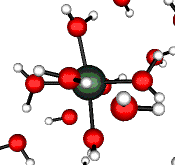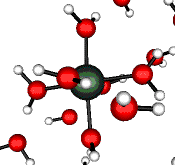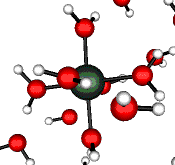
o2 (E) - Stretch
502 cm-1 (464 cm-1)
o5? (F) - Bend
320 cm-1 (281 cm-1)
o6? (F) - Bend
(225 cm-1)