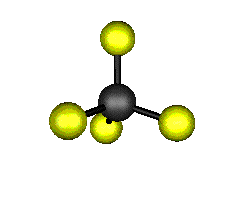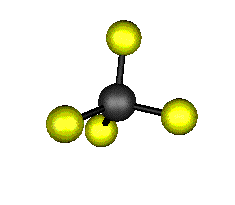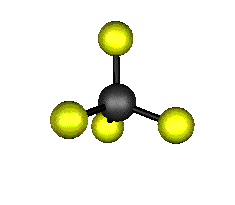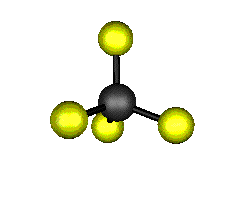
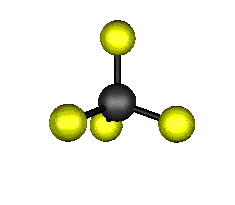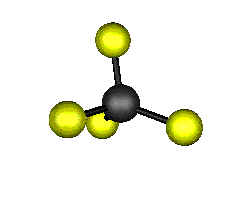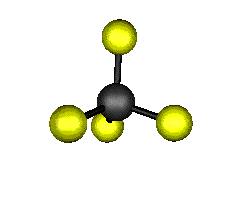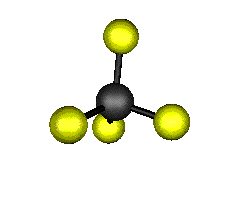
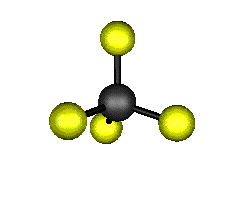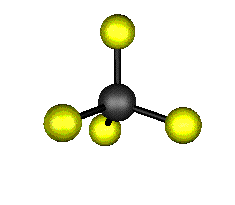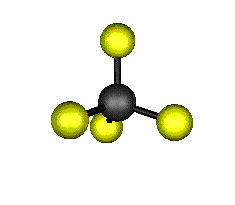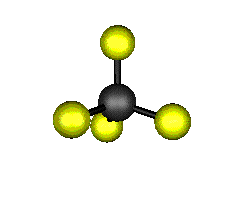
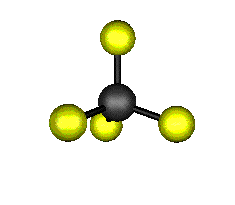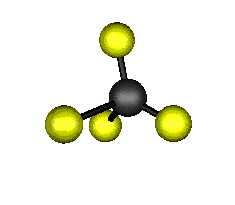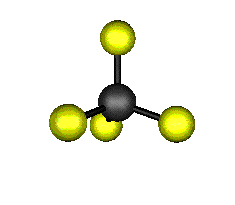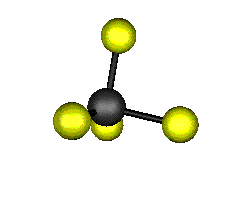
o1 (A1) - Symmetric Stretch
921.6 cm-1
o3 (F2) - Asymmetric
Stretch
1303.0 cm-1 absorbs at 7.8nm
o2 (E) - Symmetric Bend
439.9 cm-1
o4 (F2) - Asymmetric
Bend
637.9 cm-1 absorbs at 15.8nm