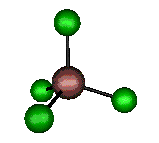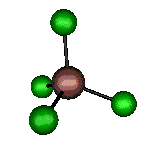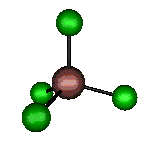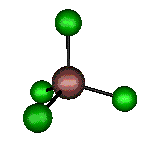
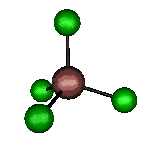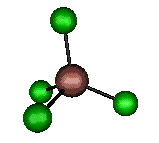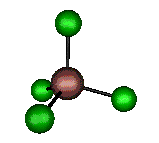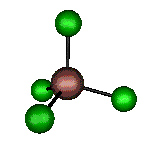
o1 (A1) - Symmetric Stretch
330 cm-1 (333.4 cm-1)
o3 (F2) - Asymmetric
Stretch
384 cm-1 (382.6 cm-1)
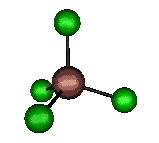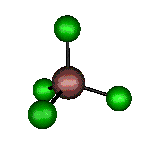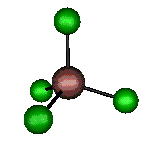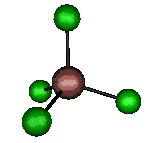
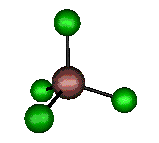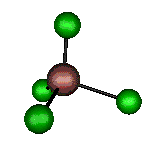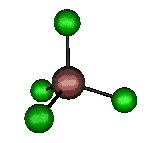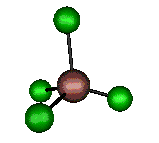
o2 (E) - Symmetric Bend
105 cm-1 (104.4 cm-1)
o4 (F2) - Asymmetric
Bend
127 cm-1 (148.8 cm-1)